
Figure 1 The morphology of corrosion of flanges
1 Physical and chemical inspection
1.1 Chemical composition analyses
Cut a sample from the corroded flange for chemical analysis, and used the American Baird DV-6 spark direct-reading spectrometer to measure its chemical composition. According to the technical requirements of the chemical composition of 304 stainless steel in the ASTM A276-2013 Standard Specifications for Stainless Steel Bars and Shapes, the Cr element content in the chemical composition of the corroded flange is lower than the standard value.
Table 1 The chemical composition of the corroded flange
| Elements | C | Mn | Si | P | S | Cr | Ni |
| The measured values | 0.077 | 0.98 | 0.25 | 0.041 | 0.021 | 17.38 | 8.04 |
| Standard values | ≤ | ≤ | ≤ | ≤ | ≤ | From 18.00 to 20.0 | From 8.00 to 11.00 |
| 0.08 | 2.00 | 1.00 | 0.045 | 0.030 | 0 |
1.2 Metallographic inspection
Took a longitudinal section sample from the corroded flange. After polishing, it was not etched. Observed it under the Zeiss metallographic microscope. The non-metallic inclusions are in accordance with the GB/T 10561-2005 Measurement Grade Standards of Non-metallic Inclusions in Steel by Microexamination. The sulfide type is grade 1.5; the alumina type is grade 0; the silicate type is grade 0; the spherical oxide is grade 1.5. Check figure 2.
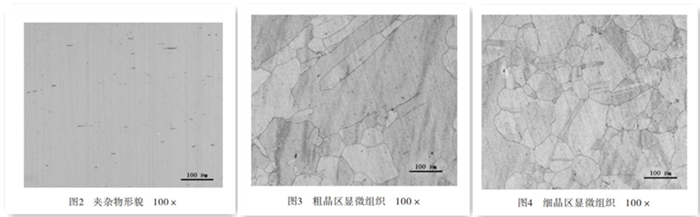
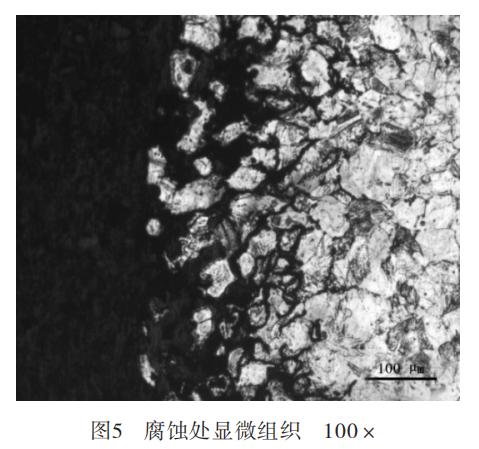
The sample was etched by an aqueous solution of ferric chloride and hydrochloric acid, and observed under a 100x metallurgical microscope. It was found that the austenite grains in the material were extremely uneven, and the grain size grade was in accordance with the GB/T6394-2002 Measurement of Average Grain Sizes of Metal. The coarse crystal area can be rated as grade 1.5 (Figure 3) and the fine crystal area 4.0 (Figure 4). Observing the microstructure of the corrosion near the surface, it could be found that the corrosion started from the metal surface (Figure 5); concentrated on the austenite grain boundary and extended to the inside of the material. The grain boundary in this area was destroyed due to corrosion, and the strength of the grain was almost completely lost. The severely corroded metal even formed powder, which could be easily scraped off the surface of the material.
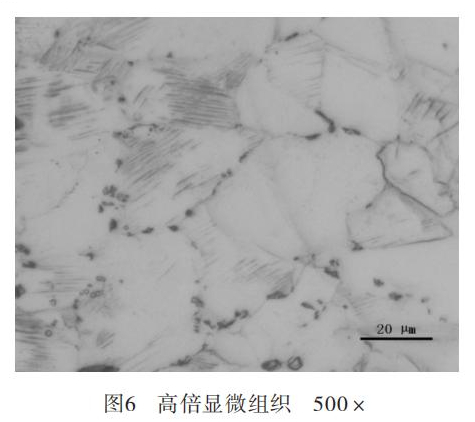
The high magnification structure of the corroded flange was observed through a 500x metallurgical microscope, and the microstructures were austenite, a small amount of ferrite and the third phase particles precipitated on the grain boundary. See figure 6.
2. Comprehensive analysis
The physical and chemical test results showed that the content of the Cr element in the chemical composition of the stainless steel flange was slightly lower than the standard value. The Cr element is the most important element that determines the corrosion resistance of stainless steel. It can react with oxygen to produce Cr oxide and form a passivation layer, which plays a role in preventing corrosion. In addition, the non-metallic sulfide content in the material is relatively high, and the accumulation of sulfide in a partial area will cause the concentration of Cr element in the surrounding area to decrease, forming a Cr poor zone and affecting the corrosion resistance of stainless steel.
It could be found that the crystalline grain size was extremely uneven by observing the crystalline grains of the stainless steel flange, and the mixed crystal grains of uneven sizes in the structure easily formed differences in electrode potential, resulting in microbattery, which led to electrochemical corrosion on the surface of the material. The coarse and fine mixed grains of stainless steel flanges were mainly related to the hot working deformation process, which were caused by the sharp deformation of the grains in the process of forging.
It could be concluded that the corrosion started from the flange surface and extended along the austenite grain boundary to the inside by analyzing the microstructure of the corrosion near the flange surface. The high-magnification microstructure of the material showed that there were more third phases precipitated on the austenite grain boundary of the material. The third phase gathered on the grain boundary would easily lead to chromium depletion at the grain boundary, causing the tendency of intergranular corrosion and greatly reducing its corrosion resistance.
The third phase in stainless steel mainly includes fine carbides (M 23C6), σ phase and δ ferrite, all of which have a great impact on the corrosion resistance of stainless steel. The formation temperature of the M23C6 precipitated phase is 450℃ to 850℃, and it mainly consists of metallic chromium carbides, most of which are distributed on the grain boundaries of the crystals; some are distributed inside the crystals and on the crystal defects, because the carbides are rich in Chromium, easily leading to chromium depletion in this area. The formation temperature of σ phase is 500℃ to 925℃, and when temperature is kept in this range, ferrite will partially or completely decompose into σ phase. The σ phase contains 42% to 50% chromium content, which is a brittle phase with good hardness and can cause a decrease of material toughness and corrosion performance. δ ferrite is high temperature ferrite, which is formed by crystallization when liquid iron is cooled to 1538°C. This phase is relatively brittle and can easily crack during processing. Pitting corrosion can easily happen.
3. Conclusion
Through a series of failure analyses of corroded stainless steel flanges, we came to the following conclusions:
(3) The low content of Cr and high content of sulfide in the material directly affect the corrosion resistance of the flange. When selecting materials, we should pay attention to using metallurgical materials of good quality.